Why Does Iodine Have a Higher Boiling Point Than Chlorine
Iodine is less reactive than chlorine. Why does iodine have a higher boiling point than chlorine.

Quick Answer Which Has The Highest Boiling Point F2 Cl2 Br2 Or I2 About Wrong
Hydrogen chloride has dipole-dipole forces so I would expect it to have greater inter-molecular forces and thus a higher boiling point.
. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. Up to 24 cash back aWhy does iodine have a higher boiling point than chlorine. Therefore the London forces between neighbouring iodine molecules are stronger than those between neighbouring chlorine molecules.
Answered by Ben W. Why does HI have higher boiling point than HCl. Explain in terms of the intermolecular forces present why ICl has a higher BOILING POINT than Cl2.
Iodine is a larger molecule than chlorine meaning it has more electrons. Boiling point of Iodine is 184C. 160 but its polar.
The boiling point of chlorine is -346 degrees C and the boiling point of iodine is 184 degrees C so iodines boiling point is massively higher Why does NO2 have a higher boiling point than CO2. This means that they require more energy to. Why does iodine chloride have a higher boiling point than Br2.
The reason Iodines boiling point is higher is because it has more electrons than Chlorine therefore there is a higher chance for an instantaneous dipole to be made and therefore more induced dipoles in other molecules. Tick one box. 13 Look at Table 1.
Why does HBr have a high boiling point. The much larger iodine atoms in HI former stronger van der Waals bonds to adjacent molecules than do the chlorine atoms in. At room temp chlorine is a gas and iodine is a solid.
When heated together chlorine reacts with iodine to form iodine monochloride ICl. 11 Why does iodine have a higher boiling point than chlorine. Iodine - Boiling Point.
Chlorine has a boiling point of 238mathrmK while hydrogen chloride has a boiling point of 188mathrmK. If you see the trend above Iodine has more electrons than Bromine hence has a greater attraction force between its atoms hence has a greater boiling point. The equation for this reaction is.
Iodine is ionic and chlorine is covalent Iodine is less reactive than chlorine The covalent bonds between iodine atoms are stronger The forces between iodine molecules are stronger 1 1 b. Because iodine and chlorine have different electronegativies the ICl molecule has a permanent dipole. Why does iodine have a higher boiling point than chlorine.
The table shows the colour and physical states. Iodine is a larger molecule with more electrons than chlorine. The boiling point of a substance refers to the temperature at which the rate of evaporation is equal to the rate.
Similar for the case of Bromine and Chlorine. Chlorine is a chemical element with atomic number. Bromine is a larger ion than chlorine and thus has stronger Van der Waals forces.
Larger amounts of energy a higher temperature are required to break these interactions and so the boiling point of HBr is higher than HCl. How do dissolved solutes affect the boiling point and the freezing point of water. The greater the attraction the more energy you have to put to break the bonds apart.
ICL has a higher boiling point than Cl2. 12 Predict the boiling point of bromine. Therefore Iodine has stronger intermolecular forces than chlorine which require more energy to break.
What is the colour of the final solution in this reaction. 1 mark A redox reaction takes place when aqueous chlorine is added to potassium iodide solution. Why is the boiling point of iodine is higher than that of chlorine.
The covalent bonds between iodine atoms are stronger. Group 7 element. This results in Iodine having a higher boiling point than chlorine.
This explains why group 7. Of chlorine bromine and iodine at room temperature and pressure. For example water boils at 100C 212F at sea level but at 934C 2001F at 1900 metres 6233 ft altitude.
1 mark Tick one box. Its pretty close to the same molecular weight 162 vs.
How To Tell Which Substance Has The Highest Boiling Point Or Freezing Point Quora

Why Is The Boiling Point Of F2 Fluorine So Low Youtube
What Is The Order Of Boiling Point Of Noble Gases Down The Group Quora
Why Does Chlorine Have A Low Boiling Point Quora
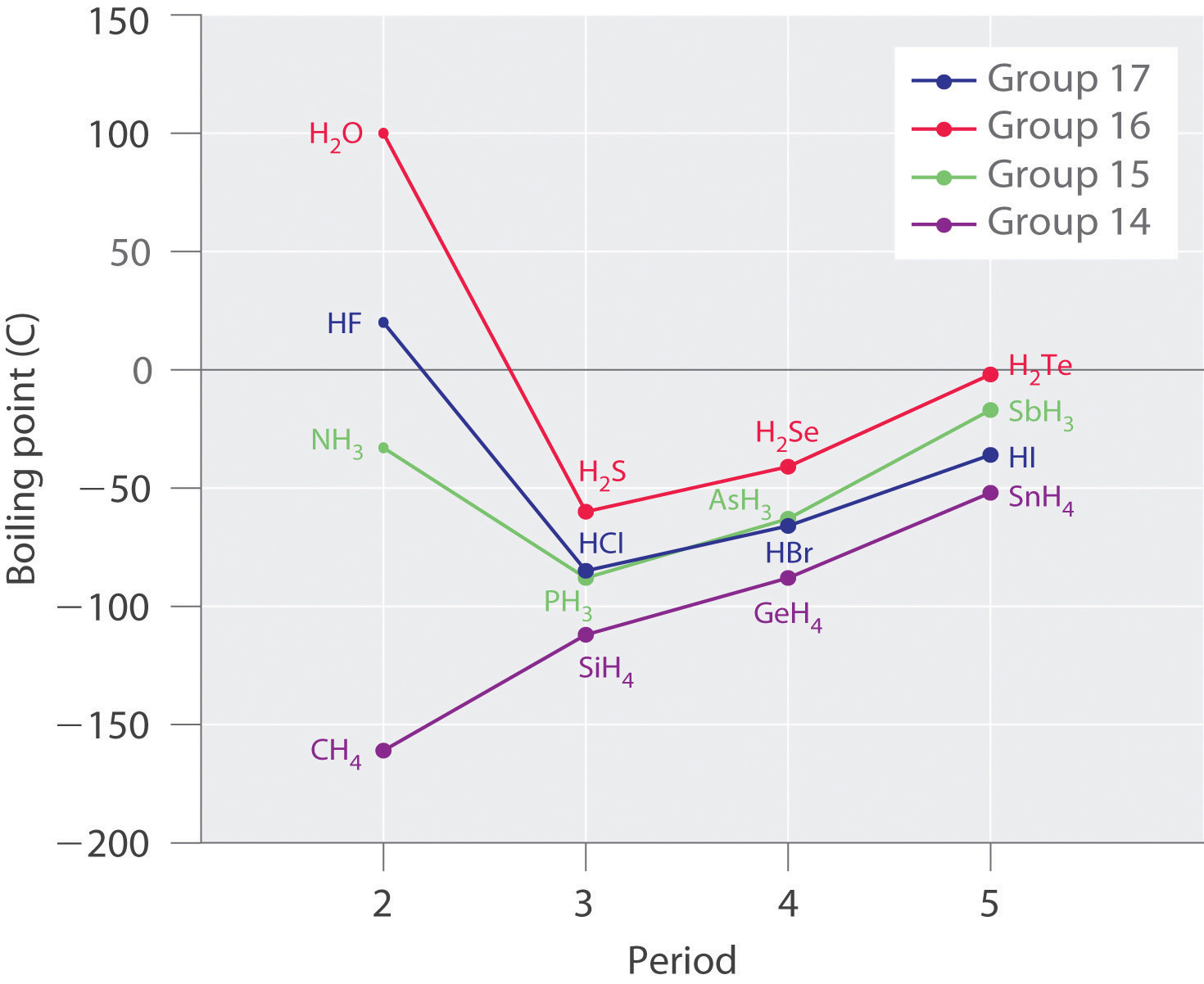
2 2 Intermolecular Forces Chemistry Libretexts

Question What Is The Boiling Point Of Hcl Seniorcare2share
Pass My Exams Easy Exam Revision Notes For Gsce Chemistry
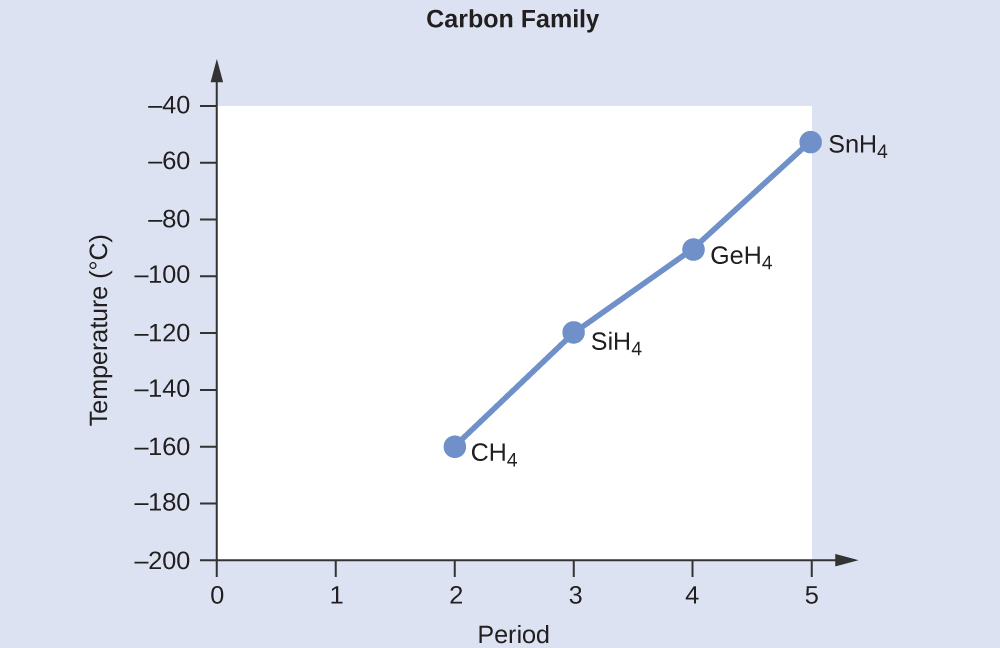
M10q2 Melting And Boiling Point Comparisons Chem 103 104 Resource Book
The Electronegativity Of Cl Is More Than Br But The Boiling Point Of Hbr Is More Than Hcl Why Quora
Why Does The Boiling Point Of A Homologous Series Increase As Molecular Mass Increases Quora
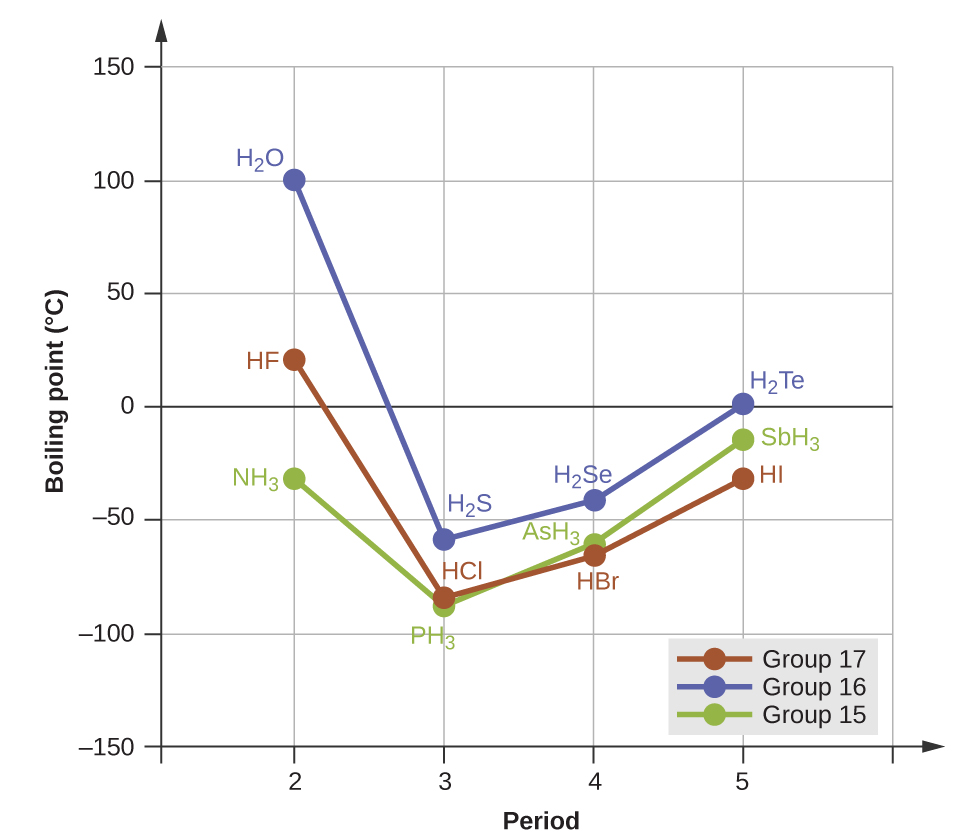
M10q2 Melting And Boiling Point Comparisons Chem 103 104 Resource Book

Comparison Of The Boiling Points Of Noble Gases Perfluorocarbons And Download Table

Haloalkanes 2 Trends In Boiling Points Explained Youtube

Arrange Each Group Of Compounds In Order Of Increasing Boiling Point Explain F2 Cl2 Br2

Melting Point Trend For Group 17 Halogens F2 Cl2 Br2 I2 Youtube

Comparison Of Boiling Points Of The Perfluoroalkanes To The Normal Download Scientific Diagram
Why Does Iodine Chloride Have A Higher Boiling Point Than Br2 Quora
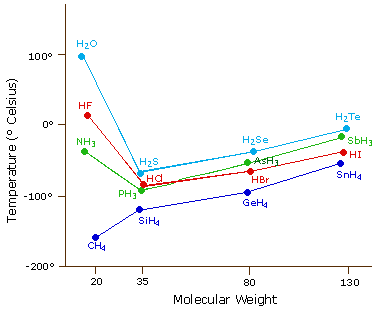

Comments
Post a Comment